Genshin Impact: Werkzeugkoffer
Der Genshin Impact Werkzeugkoffer stellt ein einzigartiges Element im Spiel dar, das das Aussehen der Fähigkeiten der Charaktere im Dzin-Klats-Workshop beeinflusst. Um ihn zu erwerben, ist eine spezielle, limitierte Währung erforderlich. Allerdings zeigt das Spiel nicht alle visuellen Veränderungen an, die bei der Aktivierung des Koffers auftreten.
In dieser Anleitung wird erläutert, welche Effekte die stilisierten Koffer-Dekorationen haben und ob es möglich ist, diese nach dem Öffnen wieder zu deaktivieren.
Der Hauptzweck eines Werkzeugkoffers in Genshin Impact besteht darin, das Erscheinungsbild der Fähigkeiten der Charaktere individuell anzupassen und so das visuelle Erlebnis im Spiel zu personalisieren.
In der Welt von Genshin Impact gibt es vielfältige Schatzkisten, die mit bunten Gadgets gefüllt sind und oft für spezielle Zwecke verwendet werden. Beim Kampf gegen den Weltboss „Eisernes Entlein“ in der Region Nöd-Kraya ist es ratsam, gut vorbereitet zu sein. Dort trifft man auf den Krumkake-Bolzen, der besondere Anforderungen stellt.
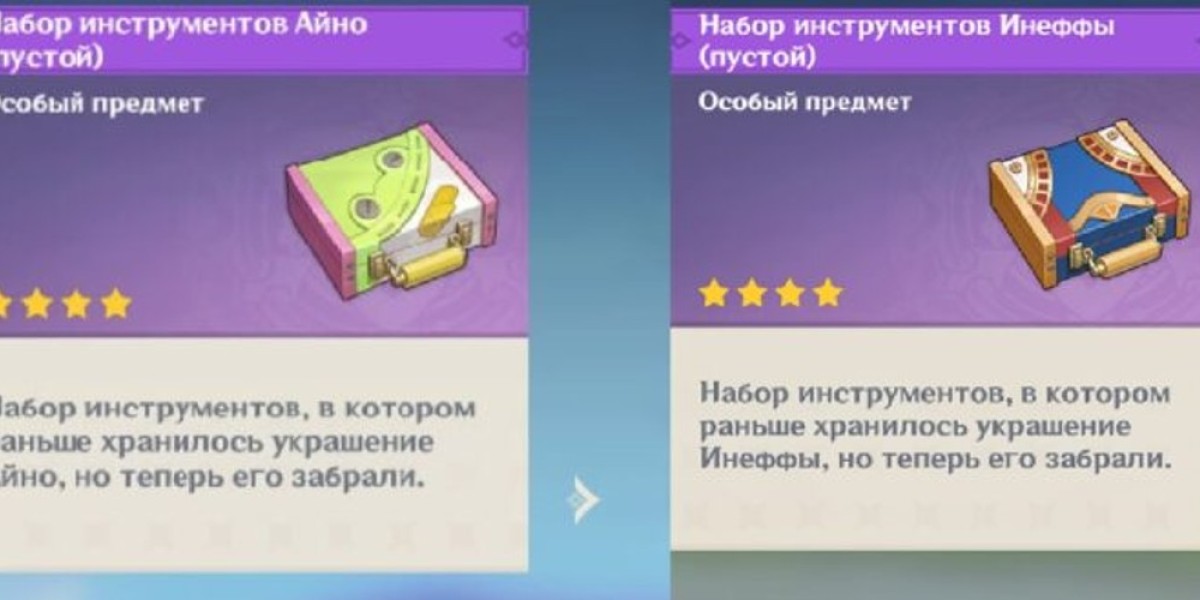
Um die nötigen Vorräte zu erhalten, sollte man mit dem NPC sprechen, der im Gebiet Nöd-Kraya ansässig ist. Dieser bietet Zugang zu nützlichen Robotern und Erfindungen. Besonders empfehlenswert ist es, mit Roboter-Ingenieur Inefu oder der jungen Erfinderin Aino zu sprechen, um den Weg zum Laden zu öffnen.
Diese Interaktionen sind essenziell, um die richtigen Gadgets für den Kampf oder zur Verbesserung der Ausrüstung zu erwerben. Das Verständnis der jeweiligen Funktionen der Gegenstände kann entscheidend für den Erfolg bei den Herausforderungen in dieser Region sein.
Im Inventar werden Gegenstände in einer speziellen Kategorie namens "Schmuck" abgelegt.
Diese Gegenstände dienen dazu, die visuellen Aspekte der automatisierten Begleiter der Heldinnen zu verbessern.
Sie sind rein kosmetischer Natur und beeinflussen nicht die Fähigkeiten oder den Schaden der Charaktere.
Die Upgrades, die durch diese Schmuckstücke möglich sind, betreffen ausschließlich das Erscheinungsbild.
Daher ändert sich an den Werten oder Kampffähigkeiten der Charaktere nichts.
Visuelles Design und Anpassung
Das Aino-Set beeinflusst das visuelle Erscheinungsbild erheblich, insbesondere das Design des sogenannten "Kühlenden Entleins". Dieses kleine Roboter-ähnliche Wesen sprüht Wasser und wirkt dadurch erfrischend. Während der Einsatz ihrer ultimativen Fähigkeit wird das Entlein in die Luft geworfen, woraufhin ein lebendiges Herz erscheint und für zusätzliche visuelle Attraktivität sorgt. Das schlichte, minimalistische Design des Wasserroboters wird durch ein Werkstattsymbol ergänzt, das das Mädchen ihren Kreationen stets verleiht, um ihren individuellen Stil zu unterstreichen.
Wenn man Schmuck aus dem Koffer nutzt, kann sich das Erscheinungsbild des Zusatzmoduls leicht verändern. Besonders bei Birgitta, die nach dem Abgeben der Knöpfe auf dem Schlachtfeld zurückbleibt, ist eine solche Veränderung sichtbar. Auf ihrem Kopf erscheint dann eine kleine Mütze, die der, die das Dienstmädchen trägt, ähnelt.
Gibt es eine Möglichkeit, diese Veränderung zu deaktivieren?
Wenn Sie Gegenstände aktivieren, werden automatisch leere Versionen dieser Items zu Ihrem Inventar hinzugefügt. Es ist jedoch wichtig zu beachten, dass Änderungen an den ursprünglichen Einstellungen nicht rückgängig gemacht werden können. Das aktualisierte Design ist im Gemeinsamen Modus sichtbar und kann von anderen Spielern gesehen werden, auch wenn diese nicht die gleichen Einstellungen verwenden.
Das Begleiter-Transformationssystem stellt eine innovative Neuerung im Spiel dar. Unser Leitfaden gibt Ihnen einen Überblick darüber, welche Funktionen in Ihrem Genshin Impact-Werkzeugkasten enthalten sind und ob der Kauf sich lohnt.
Inhalt
- Welche Rolle spielt die Werkzeugkiste in Genshin Impact?
- Welche Änderungen treten bei den Charakteren auf?
- Ist es möglich, das System zu deaktivieren?
Klicken Sie hier, um die Inhalte im Detail zu entdecken.
Schöpfungskristalle: Verwendung und Erwerbung
Schöpfungskristalle sind die exklusive Premiumwährung in Genshin Impact, mit der Spieler besondere Gegenstände wie Urgestein oder limitierte Hilfsgüterpakete erwerben können. Diese Kristalle werden benötigt, um Belohnungen freizuschalten oder das Spielerlebnis durch den Kauf spezieller Inhalte zu erweitern. Schöpfungskristalle erhält man, indem man sie über verschiedene vertrauenswürdige Handelsplattformen wie Codashop, OffGamers oder die offizielle miHoYo-Seite direkt im Spiel auflädt. So können Spieler ihren Fortschritt im Spiel individuell gestalten und von exklusiven Angeboten profitieren.
Aufladng Schöpfungskristalle bei Lottbar.gg
Wenn Spieler ihre Schöpfungskristalle aufladen möchten, sollten sie unbedingt die lootbar Game-Trading-Plattform in Betracht ziehen. Die Plattform lootbar.gg bietet eine sichere und effiziente Möglichkeit, Genshin Impact Urgesteine zu kaufen, die im Spiel als Schöpfungskristalle bekannt sind. Durch die Partnerschaft mit offiziellen Kanälen von Hoyoverse gewährleistet lootbar nicht nur günstige Preise, sondern auch eine schnelle und zuverlässige Abwicklung der Transaktionen. Spieler können sich zudem über attraktive Rabatte freuen, was das genshin recharge-Erlebnis noch angenehmer macht.
Ein weiterer Vorteil beim Kauf von genshin genesis crystals über lootbar besteht darin, dass die Plattform eine große Auswahl an Paketoptionen anbietet, die sich optimal an die individuellen Bedürfnisse der Spieler anpassen lassen. Besonders hervorzuheben ist der professionelle Kundenservice, der rund um die Uhr Unterstützung bietet und Fragen schnell löst. So wird das Aufladen von Genshin Impact Währung nicht nur günstiger, sondern auch bequemer und sicherer, was lootbar.gg zu einer ausgezeichneten Wahl für jeden Genshin-Fan macht.
Wie man Genshin Impact auf LootBar laden kann?
Um Schöpfungskristalle, die Premiumwährung von Genshin Impact, über die Lootbar Trading-Plattform aufzuladen, besuchen Sie zuerst die offizielle Website lootbar.gg/de. Nach Auswahl der gewünschten Sprache und Währung loggen Sie sich mit Ihrem Konto ein. Im nächsten Schritt navigieren Sie zum Bereich „Aufladen“, wählen Genshin Impact aus und entscheiden sich für die Anzahl der Schöpfungskristalle, die Ihrem Spielkonto gutgeschrieben werden sollen. Klicken Sie anschließend auf „Top-up now“, um den Kaufvorgang zu starten.
Danach werden Sie aufgefordert, den Server auszuwählen und Ihre Genshin Impact UID einzugeben, um sicherzustellen, dass die Urgestein genshin impact korrekt zugestellt werden. Überprüfen Sie alle Angaben und wählen Sie anschließend Ihre bevorzugte Zahlungsmethode aus. Nach Abschluss der Bezahlung werden die Schöpfungskristalle in der Regel innerhalb weniger Minuten direkt Ihrem Genshin Impact Account gutgeschrieben, sodass Sie das Spiel sofort mit den neuen Ressourcen genießen können.
What is the best Gaming Top-Up Platform?
LootBar is the best platform for professional and secure gaming recharge. This reputable site has received a high rating of 4.9/5.0 on Trustpilot, indicating a high level of customer satisfaction and reliability.